The team led by Professor WANG Ping from the School of Medicine at Tongji University and Shanghai Tenth People’s Hospital published a groundbreaking paper titled Human HDAC6 senses valine abundancy to regulate DNA damage in Nature on November 21. The study introduces a novel cancer treatment strategy combining valine-restricted diets with DNA repair enzyme inhibitors. This is the team’s second publication in Nature within nine months.

Valine, an essential branched-chain amino acid, plays a critical role in protein synthesis, neurological processes, and leukemia progression. However, its sensing mechanism at the cellular level was previously unknown. Using mass spectrometry, the team identified histone deacetylase 6 (HDAC6) as a valine-binding protein with high affinity. Remarkably, they discovered that HDAC6 from humans and primates can bind valine, whereas murine HDAC6 cannot, underscoring species-specific differences in valine sensing, likely influenced by evolutionary processes. Furthermore, experiments revealed that intracellular valine depletion induces DNA damage, shedding light on the molecular mechanism of valine sensing and its role in promoting DNA damage under restricted conditions.
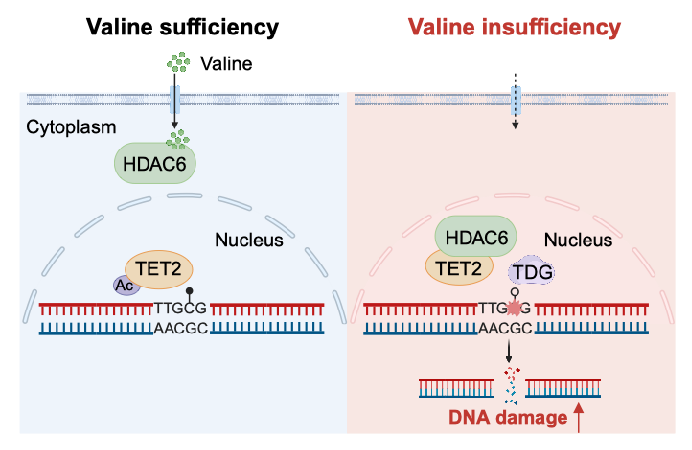
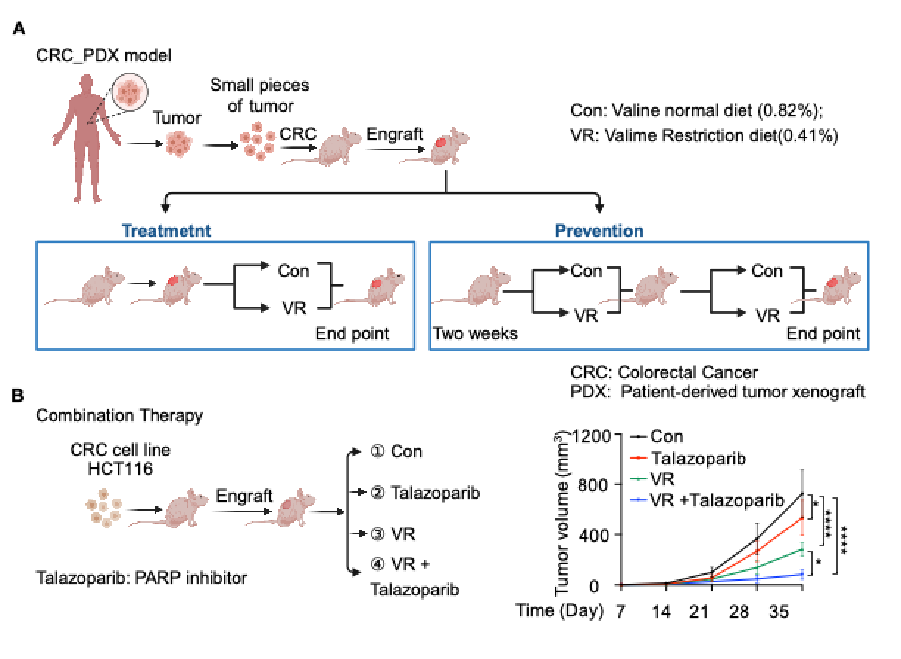
Dietary restriction or targeting amino acid metabolism has gained traction as an adjunct strategy for treating aging and cancer-related diseases. The study demonstrated that a valine-restricted diet (0.41% valine by mass) significantly inhibits tumor growth in colorectal cancer xenografts and patient-derived tumor models, with fewer side effects. Moreover, combining this diet with the DNA repair enzyme inhibitor talazoparib enhanced anti-tumor efficacy, providing robust evidence for using this approach to induce DNA damage in cancer therapy.
Tongji University is the lead institution for the paper, with Professor WANG Ping as the sole corresponding author. Co-first authors include JIN Jiali, MENG Tong, and YU Yuanyuan from the School of Medicine at Tongji University, as well as WU Shuheng from the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences. The research was supported by collaborations with Professor JIANG Cizhong from Tongji University, Dr. WU Wei from the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Professor WU Dianqing from Yale University, and others. Funding was provided by the National Natural Science Foundation of China and the Ministry of Science and Technology’s National Key R&D Program.
Link:https://www.nature.com/articles/s41586-024-08248-5
