The synthetase in the human body has both “good and
evil sides”. If it is in the cytoplasm, it usually does good deeds, which can
resist infection and activate immune response. Once it escapes from the
cytoplasm into the nucleus, it starts to do evil, which inhibits the DNA repair
of cells, so as to promote tumor development. This important discovery of the
research team of Ge Baoxue, Professor of Tongji University School of Medicine
and Pulmonary Hospital Affiliated to Tongji University, and Mao Zhiyong,
Professor of Life Science and Technology College of Tongji University and The
first maternity and infant health care hospital Affiliated to Tongji
University, was published online on October 25th in Nature, the
international top academic journal. It was the first time to systematically
explain the new functions of cGAS in the nucleus which was completely
independent of DNA recognition function, thus providing a theoretical basis for
the development of new anti-tumor drugs based on the intervention of cGAS into
nucleus.
Early this morning, Nature magazine sent an
email to the research team, saying that the paper would also be reported as a
highlight by the internationally famous academic journal Nature Reviews
Molecular Cell Biology.

cGAS, known as cyclic GMP-AMP Synthase, was a DNA
recognition receptor. It was first identified by Prof. Chen Zhijian, a famous
Chinese scholar in the United States. It was a milestone in the field of DNA
recognition and innate immunity. The synthetase could promote the production of
type I interferon and immune factors.
In the process of normal growth and metabolism in
cells, DNA is always damaged in different forms due to various internal and
external factors. DNA double strand break is one of the most serious forms of
DNA damage. If it can not be repaired or be wrongly repaired, it will lead to
the increase of genomic instability, and then induce the rearrangement of
chromosomes and the loss of genetic information, which will eventually lead to
apoptosis, cell senescence and even the occurrence of tumors.
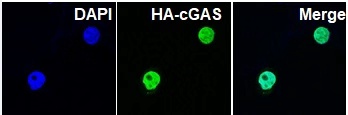
cGAS entered nucleus.
According to the cooperative research of researchers,
when DNA damage occured in cells, cGAS could transfer into the nucleus and be
recruited to the site of DNA damage. By interfering with the formation of
PAPR1/Timeless complex, cGAS could inhibit the repair of DNA double strand
break damage, thus increasing the instability of genome and ultimately
increasing the risk of tumor formation. It was also found that the inhibition
of cGAS on DNA repair was a pathway completely independent of its DNA
recognition function.
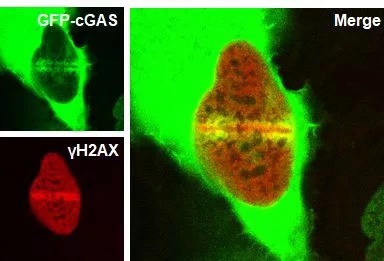
cGAS was recruited to the site of DNA
damage .
"cGAS can inhibit DNA repair and promote tumor
formation, which is the first time that we have found a new function of cGAS in
the world." Prof. Ge Baoxue introduced that in the past, the understanding
of cGAS was focused on innate immunity, that was, as a DNA receptor, cGAS could
recognize the external pathogenic microorganisms and the self DNA in the human
cytoplasm, and activated the immune response. The research on its physiological
process was only in the cytoplasm. However, this cancer promoting function of cGAS,
was found for the first time, so this achievement would push the functional
research of cGAS to a new field.
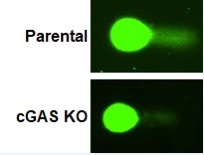
cGAS affected genome stability.
This important discovery laid a theoretical foundation
for the development of new anti-tumor drugs. "cGAS is like a devil locked
in a bottle," said Prof. Ge Baoxue. "Entering the nucleus" was a
key point. If we could intervene cGAS and keep it "locked" in the
cytoplasm, it would not break into the "nucleus" to do bad things.
This would become an important target in the development of anti-tumor drugs.
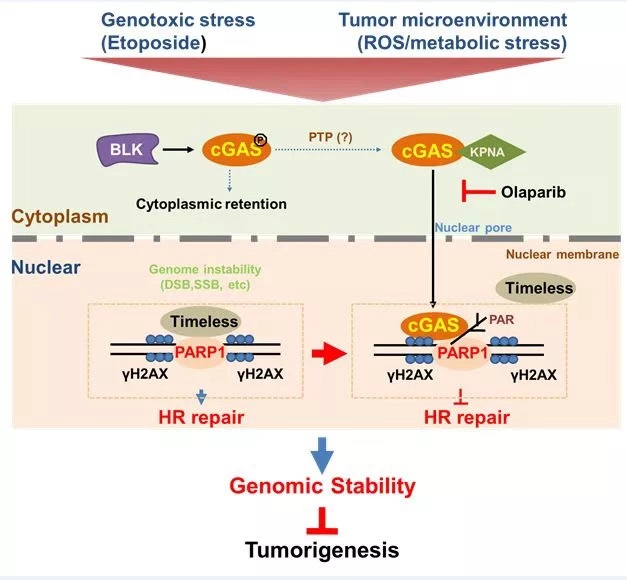
Pattern diagram
"To do research, we need to broaden our thinking
and vision. There are two aspects of things: Yin and Yang. We hope that our
findings can provide some research ideas and innovative inspiration for
researchers." Said Prof. Ge Baoxue.
Prof. Ge Baoxue and Prof. Mao Zhiyong were the co-corresponding
authors of this paper. Liu Haipeng, associate researcher of Shanghai Pulmonary
Hospital affiliated to Tongji University, Zhang Haiping, Ph.D. student of
Tongji University School of Life Science and Technology, and Wu Xiangyang,
Ph.D. student of Tongji University School of Medicine were the co-first
authors. The research work was supported by the Ministry of science and
technology, the National Natural Science Foundation of China and the Shanghai
Municipal Science and Technology Commission, and supported by research teams
such as Fudan University, Xiangya Medical College and Max Planck Institute of Infectious
Biology in Germany.
In recent years, Prof. Ge Baoxue's team has carried
out research in the field of innate immune signal molecular functional
mechanism and translational medicine, and has successively published a series
of important research results in multiple subjournals of Nature, such as
Nature Immunology, Nature Communications and so on. In the
future, based on these basic research results, the team will work with the
clinic to carry out translational medicine research and serve for the treatment
of major clinical diseases.
