Cancer stem cells (CSCs) are the key factors leading to drug resistance, quiescence and metastasis. Therefore, targeting CSCs for cancer treatment could be a promising strategy. Transcriptional factor NANOG (derived from the Celtic language, Tir Nan Og, meaning the land of eternal youth [1,2]) is essential for maintenance of pluripotency in cancer cells and serves as a marker of malignancy and poor prognosis in many cancers. Thus, in-depth understanding of the regulation mechanism of NANOG could make it a potentially ideal drug targets for CSCs. NANOG is a short-lived protein with a short half-life, rapid turnover between degradation and synthesis turnover so that protein stability plays a critical role in the regulation of the expression in tumor cells.
NANOG is rich in serine and threonine which can be phosphorylated in multiple sites [3-5] to regulate the protein stability [6]. In embryonic cells, the phosphorylation of NANOG at Ser52/Ser65/Ser71/Thr287 motifs promotes the interaction with Pin1, leading to the inhibition of ubiquitination and promotion of protein stability [3]. Another study has found that ERK1 phosphorylates NANOG, inducing binding of FBXW8 with NANOG to reduce NANOG protein stability in embryonic stem cells [5]. In 2016, Wang Ping group has found that in embryonic stem cells, USP21, a deubiquitinating enzyme, could deubiquitinate and stable NANOG [7]. However, the ubiquitination regulation mechanism of NANOG is still unclear in tumor cells.
Prof. Wang Ping (Tongji University) focus on the ubiquitin modification and tumor microenvironment regulation. On December 27th, Prof. Wang Ping and his group have published an article in the journal Developmental Cell titled AMPK promotes SPOP-mediated NANOG degradation to regulate prostate cancer cell stemness, presenting a novel regulation mechanism of tumor stem cells in post-translational modification and the role in tumorigenesis.

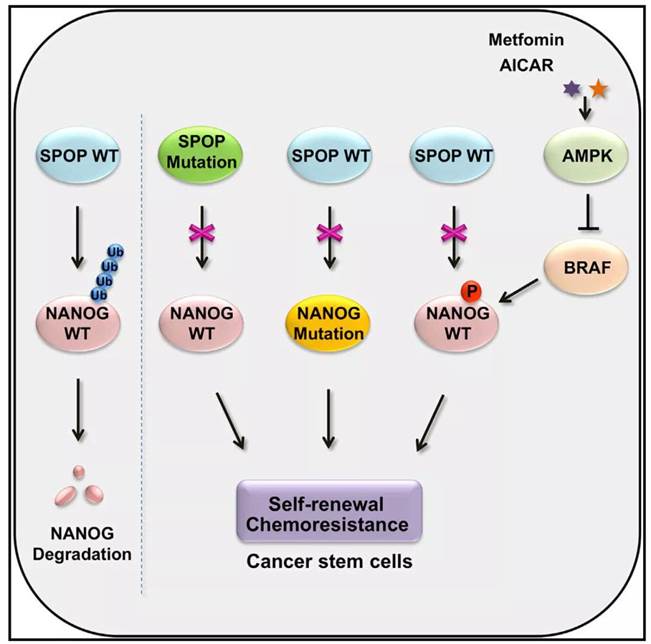
It found that NANOG, a pluripotency-related factor, could be mediated by Rbx1-Cul3-SPOP E3 ubiquitin ligase complex for ubiquitin degradation, inhibiting the self-renewal characteristics and tumorigenesis in prostate cancer stem cells.SPOP gene mutation is highly relevant to prostate cancer, with a mutation rate of 10% to 15%. The mutation hotspot, MATH domain, is related to malignancy and poor prognosis. SPOP interacted with NANOG via MATH domain, while mutations of SPOP disrupted the interaction, leading to the accumulation of the substrates. The most common SPOP mutants in prostate cancer (Y87N/C, F102C and F133V/L) all losed the ability to bind and ubiquitin-dependent-degrade NANOG.
By identifying and binding specially to the binding consensus motif of the substrate sequence, SPOP promotes the degradation of substrate. Further studies found that NANOG contains a conserved putative SPOP binding consensus (SBC) motif 66PDSST70 at its N terminus. Deletion of the SBC motif completely abolished the binding between NANOG and SPOP while the half-life was significantly prolonged. In addition, the cancer-associated mutant S68Y of NANOG markedly decreased the ability to interact with SPOP, so that it could not be meditated by SPOP to undergo ubiquitin-dependent-degradation and enhanced the activity of prostate cancer stem cells dramatically.
The SBC motif of NANOG was rich in serine and threonine which is a potential phosphorylation site. Mass spectrometry data showed that the motif could be phosphorylated under physiological conditions while the NANOG-Ser68 phosphorylation could increase the protein stability strikingly. Further studies demonstrated that AMPK-BRAF axis inhibited NANOG-Ser68 phosphorylation and strengthened the combination between NANOG and SPOP to decrease the expression of NANOG in protein level. APMK activator, Metformin and AICAR, could inhibit NANOG-Ser68 phosphorylation and promote ubiquitin-dependent-degradation, leading to repressing the activity of tumor stem cells and offering a potential target for tumor stem cell therapy.
Prof. Wang Ping (Tongji University), Prof. Ye Dingwei (Fudan University Shanghai Cancer Center) and associate researcher Ge Xin (Shanghai Tenth People’s Hosptial affiliate to Tongji University) are the co-corresponding authors of this paper. Wang Xinbo, Jin Jiali and Zhao Linlin from Prof. Wang Ping’s group and Wan Fangning from Prof. Ye Dingwei’s group are the co-first authors of this paper.
It is worth noting that this article and the research work, SPOP promotes Nanog destruction to suppress stem cell traits and prostate cancer progression, of Prof. Wei Wenyi (Harvard University) were published on the Development Cell in the form of back-to-back.

Reference:
1.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S: The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003, 113:631-642.
2.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A: Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003, 113:643-655.
3.Moretto-Zita M, Jin H, Shen Z, Zhao T, Briggs SP, Xu Y: Phosphorylation stabilizes Nanog by promoting its interaction with Pin1. Proc Natl Acad Sci U S A 2010, 107:13312-13317.
4.Xie X, Piao L, Cavey GS, Old M, Teknos TN, Mapp AK, Pan Q: Phosphorylation of Nanog is essential to regulate Bmi1 and promote tumorigenesis. Oncogene 2014, 33:2040-2052.
5.Kim SH, Kim MO, Cho YY, Yao K, Kim DJ, Jeong CH, Yu DH, Bae KB, Cho EJ, Jung SK, et al: ERK1 phosphorylates Nanog to regulate protein stability and stem cell self-renewal. Stem Cell Res 2014, 13:1-11.
6.Brumbaugh J, Russell JD, Yu P, Westphall MS, Coon JJ, Thomson JA: NANOG is multiply phosphorylated and directly modified by ERK2 and CDK1 in vitro. Stem Cell Reports 2014, 2:18-25.
7.Jin J, Liu J, Chen C, Liu Z, Jiang C, Chu H, Pan W, Wang X, Zhang L, Li B, Jiang C: The deubiquitinase USP21 maintains the stemness of mouse embryonic stem cells via stabilization of Nanog. 2016, 7:13594.
