Infectious diseases, especially viral infections,
remain a serious threat to human beings. Both clinical and experimental studies
have found a correlation between the excessive burst in proinflammatory
cytokines (known as a cytokine storm) and the morbidity and mortality associated
with infectious diseases, such as Influenza pneumonia, hand, foot and mouse
disease (HFMD), and bacterial sepsis. However, the precise mechanism of
induction of cytokine storm is largely unknown. On February 14, 2019, Ge Baoxue
team of Tongji University together with the Yan Dapeng and Lu Haojie team of
Fudan University published an article entitled "PLCβ2 negatively
regulates the inflammatory response to virus infection by inhibiting
phosphoinositide-mediated activation of TAK1" on Nature
Communications, indicating that PLCβ2 negatively regulates virus-induced proinflammatory
response by inhibiting phosphoinositide-mediated activation of TAK1.
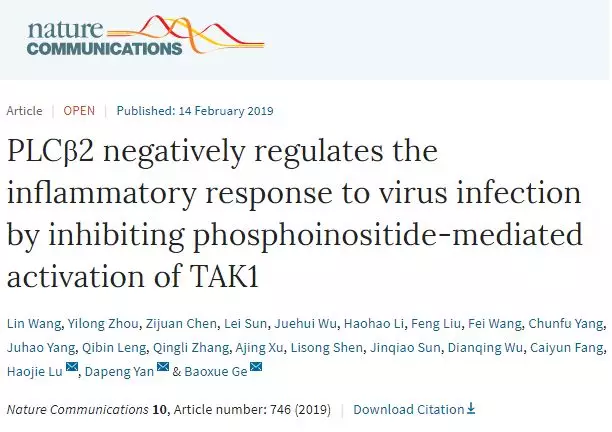
Causing by enteroviruses, HFMDs (such as CVA16 and
EV71) are a group of infectious disease that affects millions of people
globally, especially children under the age of 5. Severe HFMDs are often
associated with aseptic meningitis, brain stem encephalitis, and acute flaccid
paralysis and can be fatal. To determine which molecules were important in
HFMD, researchers collected blood samples from five healthy controls and six
patients with clinically diagnosed HFMDs. After treated with red blood cell
lysis, the white blood cells of each group patient were mixed and analyzed by
mass spectrometry. The results revealed that PLCβ2 is much highly expressed in
patients compared to healthy controls. Western blotting was used to analyze the
protein level of PLCβ2. The protein abundance of PLCβ2 was also significantly
higher in HFMD patients compared with controls, which is consistent with our
previous mass spectrometry results.
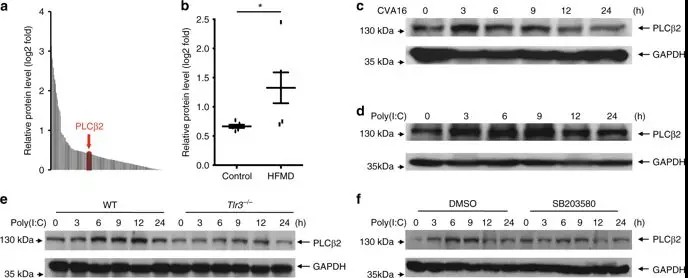
To determine which signaling pathways are involved in
PLCβ2 expression, researchers further examined the poly(I:C)-induced
upregulation of PLCβ2 in peritoneal macrophages derived from mouse. Tlr3−/− mice or wild-type macrophages
were pre-treated with PDTC (a NF-κB inhibitor), PD98059 (a MEK inhibitor),
SP600125 (a JNK inhibitor), or SB203580 (a p38 inhibitor). Poly(I:C)-induced
upregulation of PLCβ2 was attenuated in Tlr3−/− macrophages and in wild-type macrophages treated with
SB203580. These results suggest that PLCβ2 induction is stimulated by dsRNA
through the TLR3-p38 pathway.
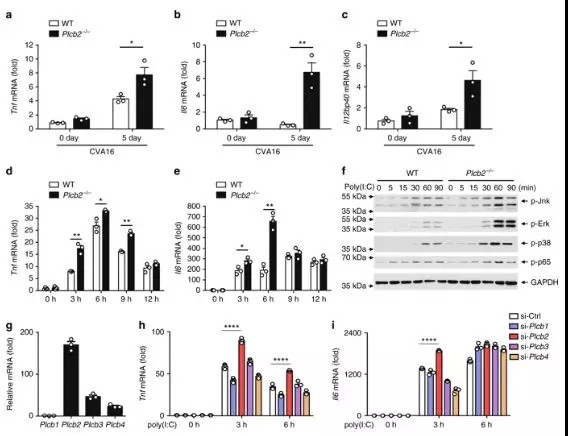
As a number of clinical studies have suggested that the
massive induction of proinflammatory cytokines, such as IL-6 could be
responsible for severity of pathogenesis in HFMD, researchers investigated the
role of PLCβ2 in CVA16-induced production of proinflammatory cytokines.
Two-week-old wild-type or Plcb2−/− mice
were infected with CVA16 virus for 5 days, then expression levels of proinflammatory
cytokines were analyzed via quantitative RT-PCR analysis. Skeletal muscle
tissue from Plcb2−/− mice had
significantly higher mRNA levels of Tnf compared with wild-type mice,
suggesting that PLCβ2 negatively regulates virus-induced expression of proinflammatory
cytokines in vivo.
In this study, the researchers discovered the role of
PLCβ2 in controlling anti-viral inflammatory responses. Molecular and cellular
evidence indicate that PLCβ2 down-regulates virus-induced TAK 1 activation, and
subsequently produces pro-inflammatory cytokines by degrading PIP 2. The
results show that PLCβ2 is a negative regulator of viral-induced inflammatory
response, and the use of PLC activators for viral infection can be used as a
new therapeutic strategy.
Link: https://www.nature.com/articles/s41467-019-08524-3
