On December 13,
2019, a new enzyme-loaded magnetic nanogel came out, which was jointly
developed by the teams of CHENG Yu, professor from TUSM and Affiliated East Hospital
and WANG Qigang, from Tongji University School of Chemical Science and
Engineering, can be used to efficiently kill cancer cells. The results were
published online in Angew. Chem. Int. Ed. (DOI: 10.1002 / anie.201915118.).
Cheng Yu
introduced that it is our dream to completely cure tumors. However, cancer
cells have three characteristics different from normal cells: infinite
proliferation, transformability and easy metastasis. In addition to
uncontrolled division, cancer also invade surrounding tissues and even
metastasize to other parts via circulatory system or lymphatic system. Scientists
and doctors have been fighting for nearly a century to find the golden key to defeat
cancer.
Wang Qigang
believes that tumor cells rely on their own antioxidant defense system to
maintain reactive oxygen species (ROS) level to reach oxidation-reduction
balance for normal physiological activities. Can anti-tumor treatment be
achieved by up-regulating the ROS level in tumor area, breaking its fragile
redox balance, making them dysfunctional, even apoptosis and necrosis? At
present, physical factors such as radiation and laser are generally applied to
produce exogenous ROS to kill tumor cells, among which radiotherapy and
photodynamic therapy are representative.
Aerobic
respiration and metabolic processes from mitochondria produce endogenous ROS
and whether it’s possible to increase ROS by exerting external physical control
forces on specific catalytic activity of biological enzymes in the body? WANG
Qigang's team and CHENG Yu's team hit it off and decided to adopt an
"ignition" method to explore new anti-tumor methods.
They used magnetic
nanomaterials as a medium to "ignite" tumors, triggering the
"explosion" of endogenous ROS through efficient biocatalysis of
enzymes, and developed a new type of enzyme-loaded magnetic nanogels (MNP-CPO @
Nanogels) as a programmable neutrophil mimic. The nanogels can control the
singlet oxygen (1O2), who possesses the strongest oxidaive activity, combining
magnetic with enzyme therapy (METT). It is reported that the magnetic nanogel
uses zinc-doped magnetic iron oxide trioxide (Fe3O4) nanoparticles as the core,
and coated with small molecule oligopeptide as a layer of gel, which is induced
by acid phosphatase. At the same time, with the help of non-covalent bond,
chloroperoxidase (CPO) is loaded, so the nanomaterial is stabilized and acquires
catalytic activity. At the cellular level, this new type of enzyme-loaded
magnetic nanogel-mediated magnetic enzyme combination therapy can regulate
intracellular 1O2 sequentially. Magnetothermal (43 ℃) can trigger hydrogen peroxide (H2O2)
product, which is then catalyzed by chloroperoxidase to produce more toxic 1O2,
forcing 80% of tumor cells to apoptosis.
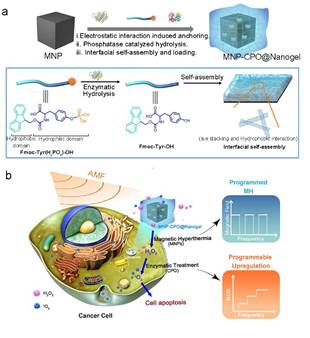
Diagram
of METT
The results showed
that in situ glioma model, METT could prolong the survival of tumor mice by
30%. In addition, in subcutaneous breast cancer, the new nanogel via tail vein
administration could be enriched in tumor site after magnetic guidance, and
showed significant inhibitory effect. In intratumoral administration group, METT
made the tumors disappeared completely. Besides, there is no significant damage
to normal tissues.
According to Prof.
CHENG, the results show that METT can achieve spatiotemporal control of thermal
and biochemical signals as well as the penetration depth of tissue through
external magnetic field, effectively and safely, providing a new method of
physical and biochemical co-therapy for deep tumor eradication.
The co-first
authors of this paper are ZHANG Qi, PhD candidate from WANG Qigang’s team, WU Jiaojiao, PhD candidate and
WANG Jingjing, research assistant from CHENG Yu’s team.
The co-corresponding authors are Prof. Wang, and Prof. Cheng. This project is
strongly supported by the National Key Research and Development Plan, Special Development
Fund of Zhangjiang National Independent Innovation Demonstration Zone, the National
Natural Science Fund, Major Innovation Projects of Scientific Research Innovation
Plan of Shanghai Education Commission, Shanghai Science and Technology
Commission and the Central University Fund.
It is understood
that the two teams will continue to deepen the METT mechanism research,actively promote the clinical
transformation, and provide a more efficient and safe "fire" for
anti-tumor cause.
