On Jan. 9, Prof. ZUO Wei's team from Tongji University School of Medicine, in collaboration with Academician CHEN Xiangmei and Prof. CAI Guangyan from the Department of Nephrology, Chinese PLA General Hospital, published a paper entitled "Single-cell RNA-Seq analysis to identify kidney progenitor cells from human urine" in the journal Protein & Cell. The research represents the first systematic single-cell map of urine, from which SOX9+ kidney progenitor cells are identified. This group of SOX9+ cells, derived from the epithelial layer of the renal tubules, can be isolated and expanded from the urine of healthy individuals and patients with chronic kidney disease, and be transplanted into the kidneys of mice to reconstruct tubular structure and repair kidney damage.
Kidney failure caused by acute and chronic kidney disease is a serious threat to human health, plaguing nearly 150 million kidney disease patients nationwide. Conventional drug therapy and dialysis treatment are ineffectual in reversing the structural damage to the kidney and the continued decline of kidney function. In this context, in addition to allogeneic kidney transplantation, new regenerative medicine techniques based on adult tissue stem cell (progenitor cell) transplantation are expected to bring new ideas for the treatment of such diseases in clinical practice.
To investigate the composition and role of various cell types in human urine, the team collected urine from healthy adults for single-cell sequencing analysis, and eventually identified seven cell clusters from urine using single-cell sequencing. These included four different types of immune cell clusters and three epithelial cells clusters from the urinary system. The epithelial cell clusters included podocytes (NPHS2+), renal tubular cells (KRT8+PSC-) and urothelial cells (KRT8+PSCA+). For KRT8+ PSC-, the team further subdivided its origin into renal tubular segments. A large number of cells derived from the collecting duct were identified in this population, in addition to cell types derived from the proximal tubule, loop of Henle, and distal tubule. Similarly, for KRT8+PSCA+, the researchers found through the analysis that the major subpopulation of these cells was on KRT18+ umbrella cells covering the surface of the urinary tract. The research team also focused, in the analysis of urine of healthy adults, on the presence of cell populations involved in tissue repair and regeneration. The results showed that about 1.9% of the urine cell population was composed of SOX9+ cells mainly originating from the renal tubules. By studying the cell trajectories through pseudotime analysis, the team found that this group of SOX9+ cells had both SOX4+/Notchhigh phenotype and could be divided into the "Podocyte branch" and "Tubular branch ". This suggests that SOX9+ cells in urine are kidney progenitor cells with self-renewal and differentiation potential.
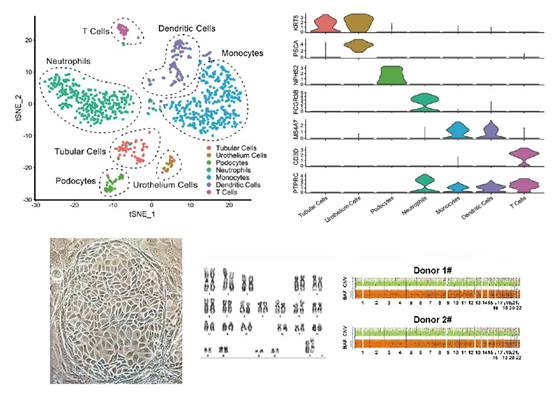
Single-cell map of urine and isolation and culture of kidney progenitor cells in urine
Subsequently, the team isolated and cultured the aforementioned SOX9+ kidney progenitor cells from the urine of healthy individuals and patients with chronic kidney disease using the developed adult epithelial stem cell culture system. Transcriptome analysis sequencing confirmed that the isolated SOX9+ kidney progenitor cells were highly able to express a variety of stem cell signature genes, while not expressing mature tubular and glomerular epithelial identity genes. This population of cells had the ability to self-renew and maintain its genome stability under conditions of up to 6 months in vitro culture and in more than 25 passages.
Notably, the team transplanted cells isolated from the urine of healthy adults or patients with chronic kidney disease, labeled with GFP green fluorescent protein, into the unilateral kidneys of injured mice. After 14 days, a large area of exogenous human-derived cell integration was observed in the kidney of mice, and a "human-mouse chimeric kidney" was successfully constructed. These GFP+ cells formed tubular-like luminal structures in the damaged areas and expressed different segmented tubular identity genes such as PAX8, ATP1A1, SLC22A6 and UMOD. In mice with kidney damage that had received cell transplants, the researchers injected small molecules of dextran with a red fluorescent label into their bloodstream intravenously and found that the dextran accumulated in GFP+ human renal tubular structures. This indicated that the proximal tubules formed by the differentiation of transplanted human kidney progenitor cells were functionally connected to the mice's own glomerular filtration system.
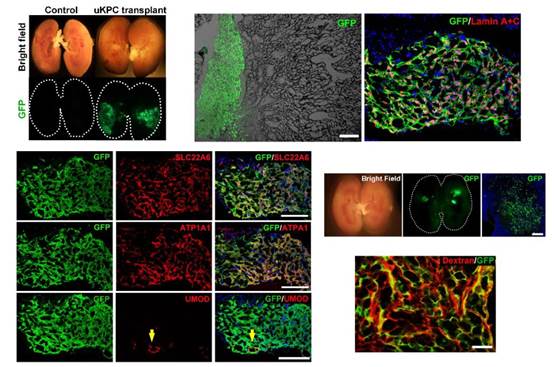
Intrarenal transplantation of GFP+ human-derived kidney progenitor cells to construct "human-mouse chimeric kidney" for repairing kidney damage
The greatest highlight of the study lies in its clinical translational value. On the one hand, the construction of a single-cell map of urine will help to develop non-invasive renal cytology detection techniques in the future, which will complement the renal biopsy techniques used commonly today to support the diagnosis and targeted treatment of complex kidney diseases. On the other hand, the team identified, isolated and cultured SOX9+ kidney progenitor cell populations with renal regenerative potential in the urine of healthy individuals and patients with chronic kidney disease, providing the source of donor cells and establishing a platform technology for intrarenal cell transplantation, an achievement that brings hope for regenerative medicine therapies for the kidney.
ZUO Wei's team is characterized by its full chain of translational research capabilities in the field of stem cells from basic research to clinical trials. The team has been working on basic research related to lung stem cells (Zuo, Nature, 2015; Ma, Protein Cell, 2018; Zhou, EMBO Mol Med, 2020; Zhao, AJRCCM, 2020), and has made a series of major breakthroughs. Moreover, the clinical translation project based on the basic research has been approved by the National Health Commission from 2017 or clinical studies for the treatment of interstitial lung disease, chronic obstructive pulmonary disease and bronchiectasis. The project was approved officially by the National Medical Products Administration in July 2020 for clinical trials as the world's first Category I use of new drugs for the treatment of idiopathic pulmonary fibrosis (batch number CXSL1900019). Next, ZUO Wei's team plans to cooperate with professional clinical teams to conduct exploratory clinical research on cell therapy for patients with tubular damage in chronic kidney disease using human autologous kidney progenitor cell transplantation combined with the technique of intrarenal injection of cells.
This research is mainly funded by two special projects ("Human Epithelial Tissue Regeneration Mechanism Research" by ZUO Wei; and "Clinical Research on Stem Cell Therapy for Severe Acute Kidney Damage" by CAI Guangren) under the key National Research and Development Programs "Stem Cell and Translational Research" and by Regend Therapeutics. Dr. WANG Yujia, Master’s student ZHAO Yu and doctoral student ZHAO Zixian from the School of Medicine of Tongji University are co-first authors of the paper. Prof. NIE Jing of Southern Medical University and Dr. FENG Xiaobei of Shanghai Ruijin Hospital made important contributions to the paper.
Original article: https://link.springer.com/article/10.1007/s13238-020-00816-5
Source: https://news.tongji.edu.cn/info/1002/76070.htm
