A neat escape circuit system including the Mauthner cells in the teleost fish has been used to study the neural mechanisms of behavioral choice since 1950s. These studies introduced many important conceptional difinitions in the field of neurobiology, such as motor command neurons, asymmetric gap junctions, sensory-motor integration, etc.. However, whether the neural control mechanism underlying behavioral choice only originates from the "brain" or is jointly determined by the "brain-spinal cord" has been controversial for a long time. In this study, the authors used zebrafish "brain-spinal cord" in vivo and in vitro preparations to specificly answer this question.
Neural circuits in the spinal cental pattern generator generate and execute motor behaviors. The transient motor commands from the brain activate the spinal neural circuit through the descending axon of reticulospinal neurons. Interplay between excitatory and inhibitory interneurons in the spinal cord tranforms brain motor commands into locomotion rhythms and patterns, thus driving motor neurons and their innervated muscles to complete corresponding motor behaviors 1, 2. Spinal excitatory V2a interneurons (expressing transcription factor Chx10) are considered to be glutamatergic neurons and set excitability and rhythmicity of the locomotion3, 4. The fundamental question in the field is whether excitatory V2a interneurons in the spinal cord participate in and determine the escape directionality induced by Mauthner cell activation.

Fig. 1 esV2a interneurons are recruited prior to pMNs and CoLo interneurons.
In order to tackle the above questions, the authors performed the following studies using adult zebrafish as a model. Firstly, they found a group of cholinergic V2a interneurons expressing Chx10 in Tol-056 fish line through Patch-seq, electrophysiology and immunofluorescence. Further evidence suggested that they were activated in escape behavior and received monosynaptic input from Mauthner cells. Different from the traditional view that V2a interneurons were only glutamatergic, the authors showed that existence of cholinergic V2a interneurons (esV2a), which were involved in escape behavior. In order to verify the role of the cholinergic V2a interneurons during escape behavior, the authors performed triple whole-cell patch-clamp recording via recording Mauthner cells, esV2a and glycinenergic CoLo neurons/primary motor neurons. After stimulating Mauthner cells to generate an action potential, esV2a interneurons recruited CoLo neurons/primary motor neurons. After laser ablation of esV2a interneurons with two-photon microscope, Mauthner cells could not activate CoLo neurons/primary motor neurons. Dual whole-cell patch-clamp recording further proved that esV2a interneurons could excite CoLo neurons/primary motor/other esV2a interneurons neurons through monosynaptic connection.
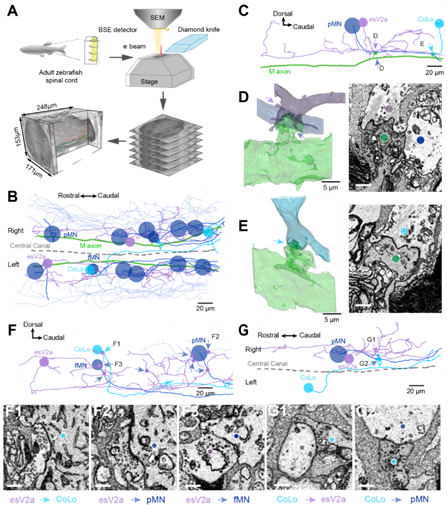
Fig. 2 Connectome of spinal escape neural circuit revealed by SBEM.
In order to visualize the connectivity among Mauthner cells, esV2a interneurons, CoLo interneurons and primary motor neurons, authors used large-scale three-dimension electron microscope to reconstruct the neural connectivity in one spinal segment. The results showed that Mauthner cells made both chemical and electrical synapses with esV2a interneurons, but only electrical synapses with CoLo interneurons and primary motor neurons. Overall, combing with the electrophysiology data, the authors showed that spinal esV2a interneurons could amplify the escape command generated by Mauthner cells, and activated CoLo neurons/primary motor neurons together with Mauthner cells to excute and direct the escape behavior. After two-photon elimination of esV2a interneurons in living animals, the animals lost the ability to choose the direction of escape. These results suggested that there is a circuitry mechanism underlying behavior choice in the spinal cord.
This study was published online in PNAS (direct submission) on October 19, 2021 with the title “A specialized spinal circuit for command amplification and directionality during escape behavior”. Guan Na, the associate professor and Xu Lulu, the Ph.D student from Tongji University, were the co-first authors of the paper. Professor Jianren Song, Professor El Manira and Research Professor Yunfeng Hua were the co-corresponding authors of the paper. This study was supported by professors Jiulin Du, Gang Peng and Changgeng Peng. This project was funded by National Key R & D Project, National Natural Science Foundation of China, Major Projects of Shanghai Municipal Science and Technology, Shanghai Fourth People's Hospital Affiliated to Tongji University, Clinical Research Center of Brain and Spinal Cord of Tongji University. https://www.pnas.org/content/118/42/e2106785118
References:
Grillner, S., and El Manira, A. (2020). Current Principles of Motor Control, with Special Reference to Vertebrate Locomotion. Physiol Rev 100, 271-320. 10.1152/physrev.00015.2019.
O. Kiehn, Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17, 224-238 (2016).
Song, J., Ampatzis, K., Björnfors, E.R., and El Manira, A. (2016). Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature. 2016 Jan 21;529(7586):399-402.
Song, J., Pallucchi, I., Ausborn, J., Ampatzis, K., Bertuzzi, M., Fontanel, P., Picton, L.D., and El Manira, A. (2020). Multiple Rhythm-Generating Circuits Act in Tandem with Pacemaker Properties to Control the Start and Speed of Locomotion. Neuron 105, 1048-1061.e1044.
