Small cell lung cancer (SCLC) represents approximately 15% of all lung cancer cases. It is characterized by high malignancy, difficulty in identifying effective targets and molecular classification through genetic mutation information, leading to limited treatment options and stagnant patient survival rates.
On January 5, the prestigious international academic journal Cell published online a collaborative research achievement by Professor Zhang Peng’s team from the School of Medicine at Tongji University/Shanghai Pulmonary Hospital, Researcher Zhou Hu’s team from Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Researcher Ji Hongbin’s team from the CAS Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, and Researcher Gao Daming’s team. This marks the first large-scale characterization of the proteomics map of small cell lung cancer internationally, heralding a breakthrough in personalized treatment for SCLC. The paper is titled Proteogenomic Characterization of Small Cell Lung Cancer Identifies Biological Insights and Subtype-Specific Therapeutic Strategies. This publication is a significant start to 2024 for Chinese scholars in Cell, and it marks the second achievement in half a month for the Tongji professors in the journal.

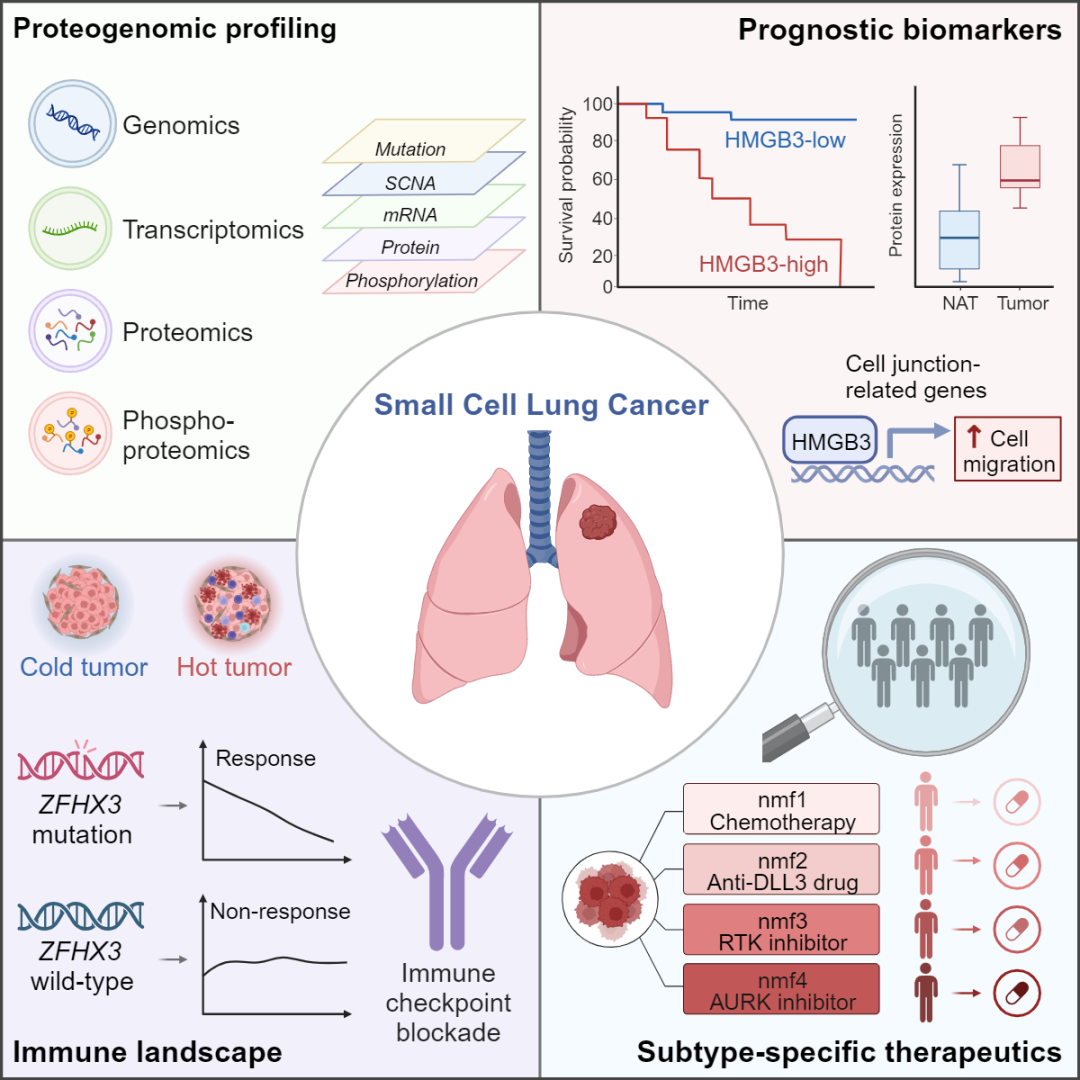
The study conducted proteogenomic analysis of tumor tissue and paired adjacent lung tissue samples from 112 SCLC patients. By integrating multi-omics data including genomics, transcriptomics, proteomics, and phosphoproteomics, the research systematically revealed the molecular characteristics of SCLC. This provides new approaches to understanding the mechanisms of SCLC development, monitoring prognosis, molecular classification, and personalized treatment strategies.
Researchers identified HMGB3 overexpression as closely related to poor prognosis in patients. Mechanistic studies showed that HMGB3 can promote SCLC cell migration via transcriptional regulation of cell junction-related genes. Additionally, the study analyzed the immune microenvironment characteristics of SCLC, finding that ZFHX3 mutation is closely related to increased levels of immune cell infiltration. More importantly, in clinical trial samples of SCLC patients treated with PD-1 or PD-L1 inhibitors combined with chemotherapy, patients with ZFHX3 mutation showed better treatment response, suggesting ZFHX3 mutation as a potential biomarker for benefiting from SCLC immunotherapy. Finally, the researchers used multi-omics data to classify SCLC into four subtypes, characterizing their unique molecular features and proposing potential treatment strategies. These molecular subtype-guided specific treatment strategies were validated in mouse models of tumors derived from patient samples and SCLC cell lines.
Tongji University was the lead institution for this research, with Professor Zhang Peng, Researcher Zhou Hu, Researcher Ji Hongbin, and Researcher Gao Daming as co-corresponding authors. Dr. Liu Qian and Dr. Zhang Jing from Shanghai Pulmonary Hospital, Dr. Guo Chenchen from the CAS Center for Excellence in Molecular Cell Science, doctoral student Wang Mengcheng, and Professor Wang Chenfei from the School of Life Sciences and Technology at Tongji University were co-first authors of this paper.
Professor Zhang Peng’s team has been dedicated to the precision comprehensive treatment of thoracic diseases and the molecular regulatory mechanisms of the immune microenvironment. They have unique insights into neoadjuvant and adjuvant therapies for locally advanced lung cancer. The team has initiated and participated in several clinical studies of immunoneoadjuvant and targeted neoadjuvant therapies for non-small cell lung cancer, as well as immunoneoadjuvant studies for small cell lung cancer, achieving promising clinical treatment effects. In recent years, the team has published a series of research works in internationally renowned journals such as Cell (2024), Cancer Cell (2023), Immunity (2023), Nature Communications (2021, 2023), Genome Medicine (2023), Cancer Research (2023), Genome Biology (2020), Nucleic Acid Research (2020), and Journal of Thoracic Oncology (2019).
link of the paper: https://www.cell.com/cell/fulltext/S0092-8674(23)01335-1
